Right now, in a clinic somewhere across Canada, a mother and her family grapple with the anguish of a devastating diagnosis. Her children, their hearts heavy with anxiety, are called back into the doctor’s office and are about to be told that their mother, who has been complaining of a nagging pain, has suspicious spots on her liver and pancreas.
As a nurse of thirty-five years, she sensed something wrong long before the diagnosis will confirm her fears. Her family, having initially dismissed her concerns, can hardly imagine that cancer is the culprit.
In moments, their world will be turned upside down. The tug-of-war between anxiety and hope, coupled with the challenge of making each day count, will shape the next two years.

This heart-wrenching moment is far from unique. Just like our family two years ago, sixteen other Canadian families will receive the same grim news each day, facing a future overshadowed by pancreatic cancer—a disease that has seen no meaningful progress in twenty years. The current five-year net survival for Pancreatic Cancer in Canada sits at only 10%, meaning out of every ten Canadians diagnosed, only one will survive beyond five years.
This reality is not just personal—it reveals systemic gaps in Canada’s pancreatic cancer response. We call for national standards in testing and treatment to ensure equitable care across provinces.
Canada is only now beginning to lay the groundwork for policies that should have been established years ago—policies that represent an overdue investment into the future of Canadians with pancreatic cancer.
These initiatives promise to bring improved trial access and engagement for Canadians in neglected rural areas and regions beyond the Cancer-care capital cities of Ontario, British Columbia, Quebec, and Alberta, but they are still years away from becoming a reality. With the average Canadian living just 3.8 months after diagnosis, the cost of these delays will be measured in thousands of lives.
Overview of Pancreatic Cancer in Canada
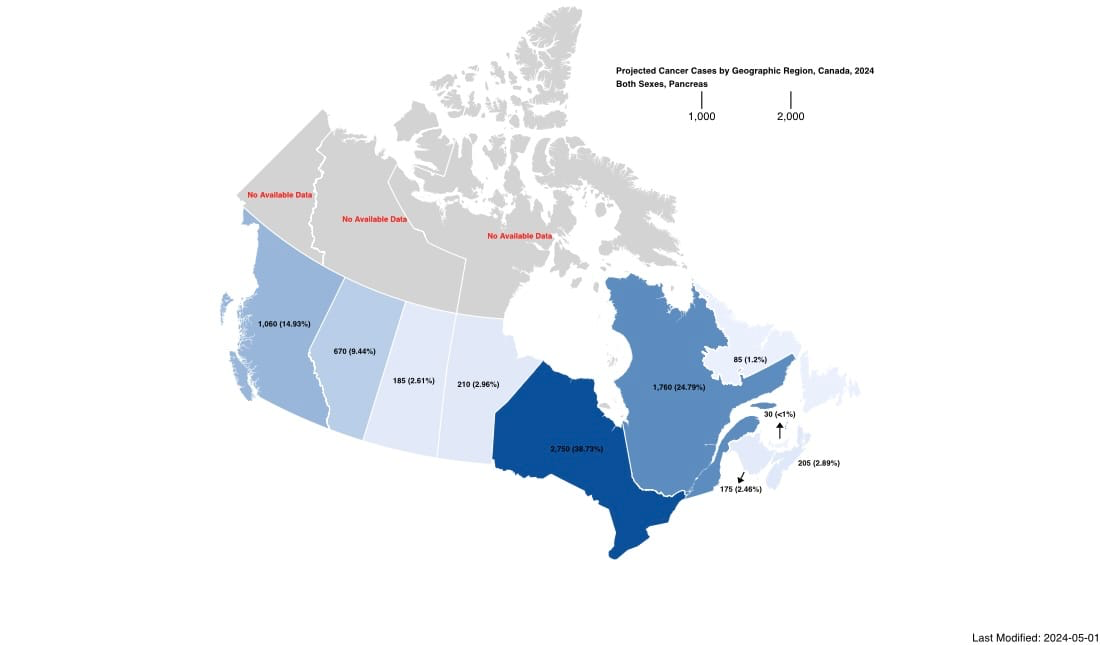
- Incidence: By 2024, it’s estimated that around 7,100 Canadians will be diagnosed with Pancreatic Cancer.
- Mortality: In the same year, 6,100 Canadians are expected to die from Pancreatic Cancer.
- The Overall Survival Rate in Canada stands at 10%.
- Age of Diagnosis: From 2011-2013, 79.5% of cases were diagnosed in those over 60, and 52% were 70 or older.
- Age-specific incidences were 20-30% higher in males than in females.
- Men: 3,800 (Diagnosed) 3,200 (Deaths)
- Women: 3,300 (Diagnosed) 2,900 (Deaths).
Barriers to Progress: Clinical Trials and Regulatory Challenges
Stagnant survival rates, limited access to clinical trials focused on precision medicine, and sluggish regulatory processes by Canadian regulatory authorities have stalled progress for decades.
With CMJA projections suggesting that pancreatic cancer will become one of the top three leading cancer deaths by the end of this year, immediate action is crucial. We cannot afford to drag our feet while countless families face devastation and heartbreak.
Urgent intervention is needed to address these profound shortcomings and ensure that every Canadian has a fighting chance for survival, regardless of their situation.
No More Riding on American Success
The stark contrast between pancreatic cancer progress in Canada and the United States reveals a critical failure in our healthcare system. The United States has led the way with annual increases in survival rates, which currently sit at 13% as of this year. While a 3% difference may not seem huge, if we acknowledge that this three percent increase took place in only the last few years, the United States is on track to reaching PANCAN’s goal of a 20% survival rate by the year 2030.

This progress is largely attributed to organizations like the Pancreatic Cancer Action Network (PANCAN), which has successfully advocated for substantial federal funding. PANCAN’s campaigns have secured millions of dollars from Congress, underscoring a robust, transparent, and visible commitment to advancing pancreatic cancer research and treatment.
Their efforts have not only driven research forward but also raised public awareness and mobilized support at the highest levels of government. It’s a common but erroneous assumption to conflate American survival rates with our own.
Canada can no longer afford to ride the coattails of American success. We must stop delaying and start taking, as Crystia Freeland would say, "bold, immediate action." Our country needs to secure substantial funding and enforce robust policy changes now—as pancreatic cancer becomes one of the top three causes of cancer-related deaths.
While we deeply respect the strides made by our neighbours, it’s time to stop riding on their coattails and build a movement of our own—one that not only reflects the unique challenges faced by people living with pancreatic cancer in Canada, but acknowledges Canada’s responsibility in the broader global stage to make meaningful progress on pancreatic cancer. We believe it’s long past time for Canadians to rally together and demand that the federal government make pancreatic cancer a national priority.

Heard enough? Click here to hop over to Our Commons, and add your signature to the growing movement.
This starts with a historic step: the first-ever e-petition at the House of Commons addressing pancreatic cancer in Canada. This petition is more than a document; it’s a call to action, a demand for equitable funding, national awareness, and a coordinated strategy to tackle one of the deadliest cancers. By signing and supporting this petition, Canadians can make their voices heard and ignite the change that’s long overdue.

Urban vs. Rural: The Unequal Fight Against Pancreatic Cancer in Canada
The disparity in pancreatic cancer outcomes between urban and rural areas significantly exacerbates this crisis. Research highlights that rural patients often face substantial barriers to accessing specialized cancer care, specifically high-volume centres and clinical trials, which are concentrated in Ontario, Quebec, Alberta and British Columbia in the countries urban cities. Princess Margaret Cancer Centre is the trophy hub of Ontario, and the largest clinical research centre in Canada, boasting more than 700+ active trials. But what about the more than thirty percent of Canadians living outside metropolitan areas?
A study in 2022 revealed that rural patients are more likely to experience delays in diagnosis and receive less advanced treatment compared to their urban counterparts, leading to worse outcomes (Cheng et al., 2022). Similarly, a study published in the Journal of Clinical Oncology found that patients in rural areas are less likely to participate in clinical trials due to geographic and logistical challenges (Sullivan et al., 2021).
Existing policies and commitments that aim to address the divide between rural and urban areas of Canada have not translated into more trial availability or better outcomes for Canadians.
While we are beginning to see precision medicine focused trials slowly increase in Canada, they are not offered in every province.
As of today's ClinicalTrials.gov search, with the exception of Ontario, British Columbia, and Quebec, there were no precision medical trials for people with Pancreatic Cancer. Zero. There is an urgent need for Canada to both fund and reform Pancreatic Cancer care.
Additionally, clinical trial participation, which has been repeatedly shown to improve survival rates and treatment efficacy (Tempero et al., 2020), is not actively promoted or integrated into standard care discussions. But again, how could they be? With no clinical trials taking place outside of urban areas, how can Oncologists pitch clinical trials when there are none?
These findings underscore the urgent need for a more equitable distribution of cancer care resources to ensure that all Canadians, regardless of location, can access the best possible treatment and support.
We’ve contacted several policy initiatives to request data on progress toward improving access to clinical trials for pancreatic cancer specifically, and will update as information is made avaialble.

Onivyde Again Denied Funding Despite Proven Survival Benefits As Negotiations Continue to Fail
Compounding the issue is the troubling decision by Canadian regulatory bodies to block funding for Onivyde. This promising chemotherapy drug, approved by Health Canada in 2017 following the Phase III Napoli Trial results, has demonstrated significant improvements in both overall and progression-free survival compared to current chemotherapy regimens.
In 2020, Pancreatic Cancer Canada rang the alarm after reporting that despite Health Canada’s approval, the Pan-Canadian Pharmaceutical Alliance (pCPA) had blocked Onivyde’s funding. This decision was made almost four years ago, and the most recent negotiations, concluded as of May 2024, still resulted in no agreement, leaving Canadians without access to this crucial third-line therapy. Onivyde has already been approved for financing in the United States, Europe, Australia, and Japan, where patients benefit from three lines of treatment. In contrast, Canadians are limited to just two.
<Key Data from the NAPOLI-3 Trial
- Median OS with standard treatment: 9.2 months
- Median OS with NALIRIFOX (Onivyde): 11.1 months
- Improvement in survival: 1.9 months
Lives Lost Due to Onivyde Negotiation Delays
Based on an annual incidence of 7100 pancreatic cancer diagnoses in Canada, over a seven-year period, approximately 49,700 Canadians were diagnosed.
With each patient losing an additional 1.9 months of survival due to the lack of access to Onivyde, we calculate the total months of life lost:
1.9 months lost per patient * 49,700 patients = 94,430 months lost
To convert months to years:
94,430 months / 12 = 7,869.17 years lost
In total, around 7,869 years of life were lost over seven years due to delays in accessing Onivyde in Canada.
The delay in funding negotiations, stretching over seven years, underscores a significant problem within Canada’s drug pricing and approval system. The pCPA, established to negotiate drug prices on behalf of federal, provincial, and territorial governments, operates with a lack of transparency and oversight. Its processes and decisions are shrouded in secrecy, leaving Canadians questioning why essential drugs like Onivyde remain inaccessible.
Hi @DougpCPA ! You said you were humbled to serve Canadians coast to coast. Yet, for almost seven years since Health Canada approved Onivyde in 2017, Canadians with pancreatic cancer are still waiting on funding. This isn't just about a drug—it's about life and death for…
— Team Heather | Heather Cutler Foundation (@TeamHeatherNL) August 13, 2024
The pCPA’s operations, which are crucial for drug pricing negotiations, are not subject to the usual scrutiny of public accountability, such as freedom of information requests or parliamentary oversight (Infoway, 2023; Macdonald-Laurier Institute, 2023).
With a survival rate of only 10% and only two chemotherapy options available, this delay in securing funding for Onivyde represents a severe neglect of the urgent needs of pancreatic cancer patients. Despite clear evidence of Onivyde’s effectiveness in extending life (Goldberg et al., 2018), the continued withholding of funding reflects a disregard for the pressing needs of Canadians battling this aggressive disease. Surprisingly, Canada’s biggest cancer organization Canadian Cancer Society has been startlingly silent on the issue.
The question arises: can the Canadian government legally intervene in the negotiations between IPSEN, the drug manufacturer, and the pCPA, which ultimately decides on funding approvals? The lack of action and transparency not only jeopardizes patients’ chances of survival but also highlights a broader issue within the Canadian healthcare system that needs urgent reform.
While the government typically does not interfere directly in these negotiations, it does have mechanisms to influence the process through public health policies and funding initiatives. Are they not aware of the urgency and impact of these delays, or are they choosing to remain passive?
Isn’t it time for the government to act decisively and end these unacceptable delays? How many more lives must be lost before we demand a system that prioritizes timely access to life-saving treatments?
This lack of proactive engagement with innovative treatments and research opportunities highlights a broader systemic issue that leaves many patients without access to potentially life-saving advancements.

The State of Funding For Pancreatic Cancer Research and Current Policy Progress
Let’s look at some of the current initiatives in Canada beyond the limited availability of clinical trials. Special thank you to Rebecca Xu at 3CTN for responding to our inquiries, in particular, historical data on the program so we could evaluate just how much the program is already increasing access for Pancreatic Cancer patients!

CRAFT Framework
Overseen by: Canadian Cancer Clinical Trials Network (3CTN)
Objective: The CRAFT framework aims to increase access to clinical trials for cancer patients in rural and remote areas across Canada and aims to establish health policies, research standards, and professional practices that establish remote trial participation as a standard part of healthcare in Canada.
More Information: Explore the CRAFT Framework
CRAFT is an exciting investment in meaningful change for Canadians in rural areas. Even greater possibilities could lie ahead in the future where remote trial participation could be an international standard, enabling patients to join trials outside Canada?
Exciting yes but still far off. The timeline for when it will benefit those with pancreatic cancer remains uncertain—and far from immediate.
Canadians urgently need access to precision medicine trials that should have been available years ago. How much longer must we wait to see real results?

Health Canada's Clinical Trial Modernization
Health Canada is in the early stages of overhauling the clinical trials framework, working through consultations and proposals. Part II of their consultation paper lays the groundwork for Decentralized Clinical Trials (DCT), trials that make use of remote technologies such as video conferencing, mobile health apps and home visits to allow trial participants to be located outside of primary research centres. They’ve also published mock-up’s and sought public feedback on a planned Clinical Trial Search Portal, which apparently takes years to implement.
Find Out More: Visit Health Canada’s Consultation Page
The proposals represent another exciting investment in future Pancreatic Cancer patients. However, Given the historical pace of such updates, it could be several years before any changes are finalized and put into practice.

CCS Breakthrough Team Grants: Transforming Low-Survival Cancers
Lead Researcher: Dr. Steven Gallinger
Location: Toronto, Ontario
Grant Sponsors: Canadian Cancer Society, Brain Canada, Canadian Institutes of Health Research (CIHR), Cancer Research Society, Lotte & John Hecht Memorial Foundation
Research Focus: Screening, early detection, disease biology, new therapies, personalized treatments
Objective: To create a comprehensive research program aimed at improving survival rates.

This initiative funded by the Canadian Cancer Society and it’s partners seeks to build a broad strategy that includes developing better screening methods, understanding the biological mechanisms of the disease, and accelerating the introduction of new, personalized therapies. The program leverages existing successes to establish a more integrated approach in hopes of combating pancreatic cancer across Canada.

What Canada Can Do in the Meantime
While we welcome updates to outdated policies and the development of new frameworks to address the future needs of Canadians with pancreatic cancer, those currently facing the disease need support as well. There are clear actions the Canadian government can take to provide immediate assistance.
- Increase Funding for Research: Boost funding for pancreatic cancer research to accelerate the development of new treatments and improve early detection methods. Targeted grants and incentives for innovative research such as the team led by Dr. Steven Gallinger could drive faster progress.
- Expand Access to Existing Clinical Trials: CRAFT doesn’t have to take years, but it will. Implement temporary measures to expand access to existing clinical trials by funding mobile units or telemedicine platforms that allow remote participation. Partner with private and public organizations to facilitate broader recruitment and support for patients in rural areas.
- Support for Patients and Families: Provide financial and logistical support for patients and their families, including assistance with travel, accommodation, and other expenses related to treatment and clinical trial participation.
- Increase Public Awareness and Education: Launch national awareness campaigns to educate the public and healthcare professionals about pancreatic cancer, its symptoms, and the importance of early detection. This could lead to earlier diagnosis and better outcomes.
- Streamline Access to Experimental Therapies: Establish pathways for expedited approval and access to promising experimental therapies and treatments currently available in other countries.
- National Pancreatic Cancer Organizations Must to Advocate for More Federal Funding: Currently, national organizations like Craig’s Cause, Pancreatic Cancer Canada, and the Heather Cutler Foundation focus on various aspects of pancreatic cancer, such as advocacy, research funding, policy, and awareness. One of these organizations must take the lead in pushing for more substantial federal support to advance research and improve outcomes.
What YOU can do
Sign Our Petition on Change.org
Our petition on Change.org is finally live and collecting both signatures and feedback! We’d love for you to get on board and urge the House of Commons to commit to the future of Canadians diagnosed with Pancreatic Cancer.

Additionally, we are advocating for somatic testing to be made available free of charge for all Canadians. If an American organization like PANCAN can offer this service to Canadian citizens at no cost, there is no reason why Canada cannot do the same.
Questions and Answers
How does Canada’s pancreatic cancer survival rate compare to the United States?
Canada’s pancreatic cancer survival rate is 10%, while the United States has reached 13%. This three percent difference reflects significant advancements in the U.S., which is on track to achieve a 20% survival rate by 2030, demonstrating the potential impact of prioritizing research and access to treatment.
What is the projected incidence of pancreatic cancer in Canada for 2024?
✅ Verified Source
In 2024, it is estimated that approximately 7,100 Canadians will be diagnosed with pancreatic cancer, and 6,100 will lose their lives to the disease. These figures highlight the urgent need for progress in early detection, treatment access, and patient support.
How does pancreatic cancer affect different age groups in Canada?
✅ Verified Source
Between 2011 and 2013, 79.5% of pancreatic cancer diagnoses occurred in Canadians over the age of 60, with 52% of cases in those aged 70 or older. Incidence rates were 20–30% higher in males than females, underscoring the disease’s disproportionate impact on older populations and men.
What is the CRAFT framework and how does it aim to improve pancreatic cancer care in Canada?
The CRAFT framework, led by the Canadian Cancer Clinical Trials Network (3CTN), focuses on expanding access to clinical trials for Canadians in rural and remote areas. Its goal is to make remote trial participation a standard component of healthcare, ensuring equitable access to innovative treatments across the country.
How has the delay in funding for Onivyde affected pancreatic cancer patients in Canada?
The seven-year delay in securing funding for Onivyde has resulted in an estimated loss of 7,869 years of life for Canadians with pancreatic cancer. This figure is based on the drug’s ability to extend survival by an average of 1.9 months per patient, emphasizing the impact of delayed access to effective treatments.
What is the current state of clinical trial availability for Canadians with pancreatic cancer?
Clinical trials focused on precision medicine for pancreatic cancer are currently limited to Ontario, British Columbia, and Quebec. This leaves Canadians in other provinces without access to these potentially life-saving trials, highlighting a significant gap in the availability of advanced treatments.
What role does the Pan-Canadian Pharmaceutical Alliance (pCPA) play in access to new pancreatic cancer treatments?
The Pan-Canadian Pharmaceutical Alliance (pCPA) negotiates drug prices for governments across Canada but operates with limited transparency and accountability. These inefficiencies have caused delays in accessing critical treatments like Onivyde, despite its proven ability to improve survival rates for those living with pancreatic cancer.
What is the CCS Breakthrough Team Grants program and how does it aim to improve pancreatic cancer outcomes?
The CCS Breakthrough Team Grants program, led by Dr. Steven Gallinger, is a major research effort to improve survival rates for pancreatic cancer. Its focus areas include screening, early detection, disease biology, the development of new therapies, and personalized treatment approaches.
How does Health Canada’s Clinical Trial Modernization proposals aim to improve access to trials?
Health Canada’s Clinical Trial Modernization initiative seeks to transform the country’s clinical trials framework through decentralized trials and a Clinical Trial Search Portal. While these advancements promise to expand access, they are still in development and years away from implementation.
What immediate actions does the article suggest Canada can take to improve pancreatic cancer outcomes?
To improve pancreatic cancer outcomes, the article emphasizes the need to increase research funding, expand access to clinical trials, provide better support for patients and families, launch national awareness campaigns, streamline access to experimental therapies, and secure more federal funding through national organizations.
Reference List 📖
- Canadian Cancer Society. “Pancreatic Cancer Statistics.” Canadian Cancer Society, 2024.
- Cheng, Lina, et al. “Study on Rural and Urban Disparities in Pancreatic Cancer Outcomes.” Canadian Medical Association Journal, vol. 196, no. 18, 2022, E615.
- Saadat, Lily V et al. “Treatment Patterns and Outcomes in Pancreatic Cancer: A Comparative Analysis of Ontario and the USA.” Annals of surgical oncology vol. 31,1 (2024): 58-65. doi:10.1245/s10434-023-14375-6
- Infoway. “Canada Health Infoway.” Infoway, 2023.
- Macdonald-Laurier Institute. “Pan-Canadian Pharmaceutical Alliance Lacks Transparency and Accountability: Nigel Rawson.” Macdonald-Laurier Institute, 2023.
- Goldberg, et al. “Onivyde as a Treatment for Advanced Pancreatic Cancer.” Journal of Clinical Oncology, vol. 36, no. 15_suppl, 2018, pp. 4003-4003.
- The Princess Margaret Cancer Foundation. “Clinical Trials.” The Princess Margaret Cancer Foundation. Accessed 13 Aug. 2024.